новый блог

Запах является одновременно чувственным опытом и воспринимаемой мерой качества.
В ограниченном пространстве салона автомобиля «запах новой машины» от пластика не является символом роскоши — напротив, он часто становится основным источником жалоб потребителей.
В этой статье, основанной на инженерной практике, систематически рассматриваются источники, механизмы, аналитические методы и контролируемые стратегии, связанные с запахом. Она призвана помочь инженерам-материаловедам в
снижение рисков возникновения запахов у источника при проектировании материалов для внутренней отделки автомобилей
.
Откуда берётся запах в пластике?
Пахучие молекулы в пластиковых материалах в основном существуют в форме летучих органических соединений (ЛОС), которые выделяются в воздух через
три основных механизма
:
1. Распространение:
Непрореагировавшие мономеры и малые молекулы мигрируют из внутренней части материала на его поверхность. ЛОС в пластике подчиняются второму закону диффузии Фика.
Например, в полипропилене (ПП) коэффициент диффузии альдегидов составляет приблизительно 10⁻⁹ см²/с. При 23°C достижение равновесной поверхностной концентрации может занять до 48 часов. Однако при повышении температуры до 60°C, что сопоставимо с летней температурой в доме, скорость диффузии может увеличиться в 3–5 раз.
2. Десорбция:
Молекулы ЛОС, адсорбированные на поверхности материала, выбрасываются в окружающий воздух.
3. Миграция:
ЛОС также могут мигрировать из таких добавок, как пластификаторы, смазочные материалы или остаточные растворители.
Как работает человеческий нос: от молекул до мозга
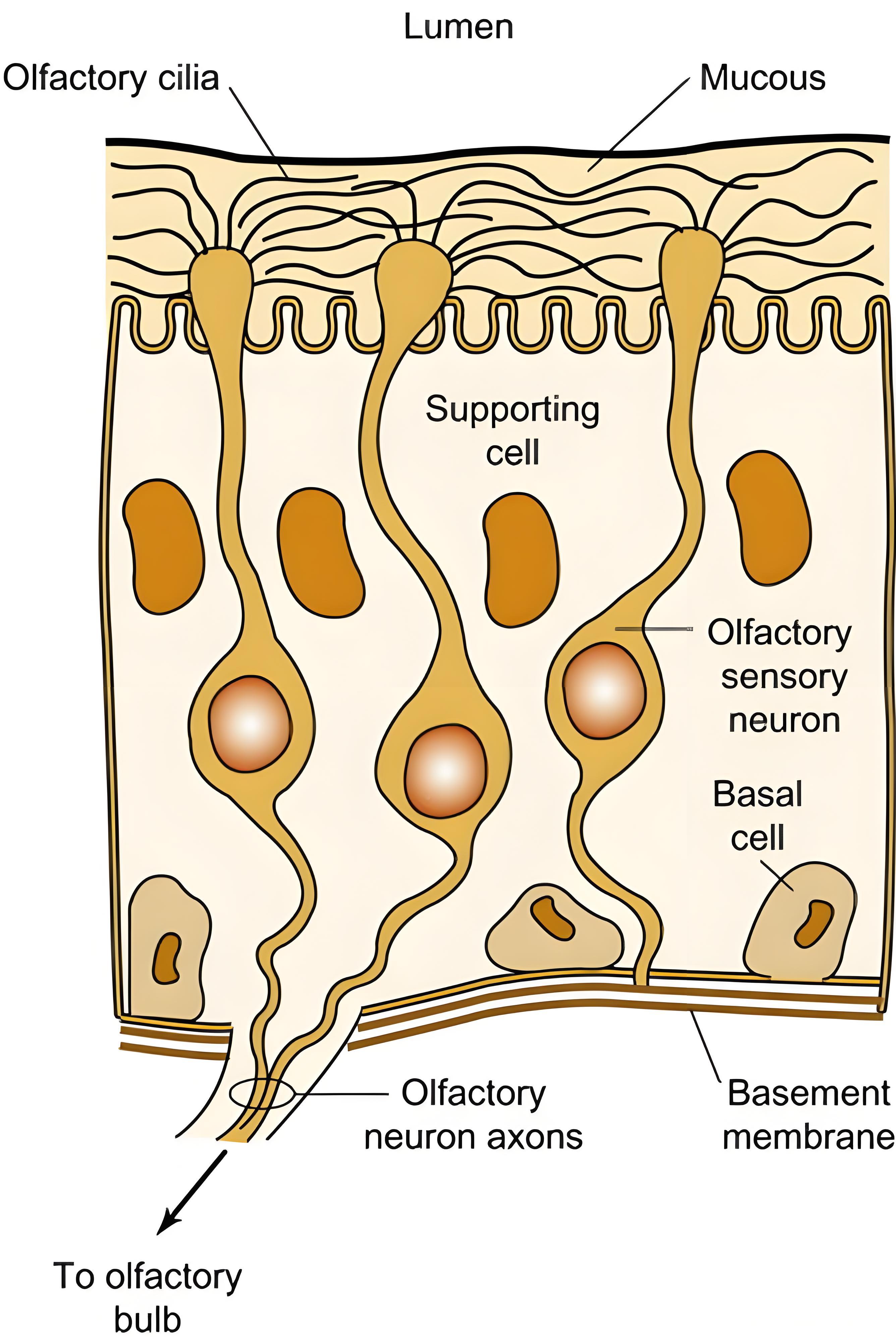
Когда летучие молекулы, выделяющиеся из пластика, такие как нонаналь и деканаль, попадают в носовую полость, начинается высокоточный процесс распознавания на микроуровне. В обонятельном эпителии (площадью примерно 5 см²) плотно распределено около 350 типов обонятельных рецепторных белков. Эти рецепторы действуют как «молекулярные замки», каждый из которых распознаёт определённые «ключи» к запахам.
Возьмём в качестве примера (E)-2-ноненаль: его углеводородная цепочка связывается с обонятельным рецептором OR51E2 с энергией связи -8,7 ккал/моль. Это специфическое взаимодействие запускает открытие ионных каналов, генерируя электрические сигналы. Передача обонятельного сигнала происходит по принципу «замка и ключа»: после связывания одоранта с рецептором, сопряжённым с G-белком (GPCR) на ресничках, он активирует вторичный мессенджер цАМФ, что приводит к деполяризации клеточной мембраны. Полученный сигнал передаётся по обонятельным нервным волокнам в обонятельную луковицу, где митральные и пучковые клетки обрабатывают его и проецируют в кору головного мозга.
Каждый обонятельный сенсорный нейрон экспрессирует только один тип рецепторов, однако благодаря комбинаторному кодированию система способна различать десятки тысяч различных запахов. Например, смесь альдегидов, выделяемых полипропиленом (ПП), может активировать такие комбинации рецепторов, как OR1A2 и OR2J3.
Этот механизм биологического распознавания служит эталоном для оценки запахов материалов. Например, когда концентрация ДЭГФ, выделяемого из искусственной кожи на основе ПВХ, превышает 2200 мкг/м³, его молекулы связываются с рецепторами OR3A4 и вызывают восприятие «резкого» запаха — именно того порога, которого стремятся избежать дизайнеры автомобильных интерьеров.
Понимая механизмы взаимодействия молекул и рецепторов, лежащие в основе обоняния человека, инженеры-материаловеды могут проводить обратную разработку составов со слабым запахом на основе «карты обонятельного восприятия человека».
Типичные запахи и их источники в различных видах пластика
| Тип полимера |
Описание типичного запаха
|
Основной источник/ Вещество или механизм |
Дополнительные примечания
|
|
Полиэтилен (ПЭ)
|
Восковой, маслянистый, легкое раздражение
|
Антиоксидантная деградация (например, BHT → фенолы), окислительное расщепление (альдегиды)
|
Запах становится более заметным при более высоких температурах обработки.
|
|
Полипропилен (ПП)
|
Слегка сладковатый, легкий маслянистый запах
|
Продукты окисления (алкилальдегиды, кетоны), остатки антиоксидантов
|
Обычно слабый запах, может усилиться после модификации
|
|
Полистирол (ПС)
|
Сладкий, ароматный, сильное раздражение
|
Остаточный мономер стирола, продукты разложения (толуол, этилбензол)
|
HIPS (резино-модифицированный полистирол) имеет более сложный запах
|
|
Акрилонитрилбутадиенстирол (АБС)
|
Острый, пряный, слегка кислый
|
Остаточный акрилонитрил, стирол, окисленный бутадиен, эмульгаторы
|
Сильное термическое разложение усиливает интенсивность запаха.
|
|
Поливинилхлорид (ПВХ)
|
Запах пластика, похожий на чернила, раздражающий
|
Пластификаторы (например, фталаты), разложение стабилизаторов, HCl
|
Низкая термостойкость; запах усиливается после разложения
|
|
Полиуретан (ПУ)
|
Рыбный, аминоподобный, сильное раздражение
|
Остаточные изоцианаты, продукты гидролиза (амины)
|
Инкапсулированные изоцианаты могут помочь уменьшить запах
|
|
Полиамид (ПА6/ПА66)
|
Жареный, с запахом аммиака
|
Конечные амины цепи, окисление, термическая деградация (например, капролактам)
|
Гидролиз после поглощения влаги также может вызывать запах.
|
|
Полиэстер (ПЭТ/ПБТ)
|
Слабый запах гари, кислый
|
Продукты разложения (бензойная кислота, фталевая кислота), остаточные растворители
|
Высокотемпературное литье под давлением имеет тенденцию выделять более сильный запах.
|
|
Поликарбонат (ПК)
|
Горький, фенольный, слегка острый
|
Остаточный БФА, разложение карбоната (на основе фенола)
|
Инкапсулированные антиоксиданты могут помочь уменьшить запах
|
|
Полиметилметакрилат (ПММА)
|
Слегка раздражающий, эфироподобный, приемлемый
|
Остаточный ММА, термическая деградация (небольшие эфиры)
|
Высокочистый ПММА практически не имеет запаха.
|
|
Полиоксиметилен (ПОМ)
|
Неприятные, раздражающие газы
|
Формальдегид, летучие вещества ацетального типа
|
Запах, выделяемый в основном при высокотемпературном литье под давлением
|
|
Фторполимеры (например, ПТФЭ)
|
Почти без запаха, с легкой восковой нотой
|
Практически нет выбросов ЛОС
|
Очень слабый запах, подходит для высококачественных внутренних работ
|
Механизмы образования запаха
Запах пластика не появляется из ниоткуда — он постепенно образуется в процессе
обработка, хранение и использование
.
К основным механизмам относятся:
1. Термическая деградация:
Высокие температуры обработки вызывают разрыв молекулярных цепей, что приводит к образованию низкомолекулярных пахучих соединений (например, альдегидов).
| Полимер |
Продукты термической деградации
|
|
Полиамид 66 (ПА66)
|
Циклопентанон, пиридин, циклический имид, амиды, карбоновые кислоты, капролактам
|
|
Полиэтилен (ПЭ)
|
Кетоны, карбоновые кислоты, фураноны, кетокислоты
|
|
Поли(оксид этилена–оксид пропилена–оксид этилена)
|
Формиатные эфиры, ацетатные эфиры, карбоновые кислоты, альдегиды
|
|
Поли(L-лактид) (PLLA)
|
Лактид, молочная кислота, лактоил-молочная кислота
|
|
Полиметилметакрилат (ПММА)
|
Мономер метилметакрилата
|
|
Силиконовый каучук (полисилоксан)
|
Циклические олигомеры
|
|
Полистирол (ПС)
|
Стирол, стиролакрилонитрил, трет-бутилбензол, α-метилстирол, BHT (бутилированный гидрокситолуол)
|
|
Полисульфидный каучук
|
1,3,6,7-диоксодитиепан, другие циклические продукты распада
|
2. Окислительная деградация:
Антиоксиданты или продукты окисления полимеров вызывают неприятные запахи (например, продукты окисления BHT).
Полиамид (ПА66): Термоокислительная деградация приводит к образованию соединений циклопентанона, таких как 2-этилциклопентанон, концентрация которых может достигать 0,3 мкг/г после старения при 100 °C в течение 300 часов, вызывая «медицинский» запах.
3. Фотостарение:
УФ-излучение вызывает разрыв полимерной цепи, в результате чего высвобождаются низкомолекулярные газы.
4. Остатки переработки:
Остаточные катализаторы или растворители, которые не были полностью удалены.
Полиуретан (ПУ): Аминовые катализаторы, такие как триэтиламин, имеют очень низкий порог обоняния (0,67 мкг/м³) и являются основной причиной характерного «рыбного» запаха пенополиуретана.
Как Ана лиз Запах пластика?
С Распространенные методы тестирования и оценки пластика О дорс Включать:
|
Метод испытания
|
Основной принцип
|
Результаты вывода
|
Приложения
|
|
Тест на сенсорное обоняние
|
Персонал субъективно обоняет и оценивает образцы с помощью обоняния.
|
Шкала интенсивности запаха (например, шкала от 1 до 6)
|
Предварительная проверка материала, сенсорная оценка конечного пользователя
|
|
Стандартный тест VDA 270
|
Образец нагревают при постоянной температуре, чтобы высвободить запах, затем вдыхают его.
|
Оценка запаха (немецкая шкала)
|
Тестирование запаха материалов салона автомобиля
|
|
ГХ-МС (газовая хроматография-масс-спектрометрия в парофазном режиме)
|
Газы в парофазном пространстве собираются и разделяются с помощью хроматографии; масс-спектрометрия для идентификации и количественного определения
|
Типы и концентрации ЛОС (мкг/м³)
|
Точная идентификация источников запаха
|
|
ТД-ГХ-МС (термодесорбционная ГХ-МС)
|
Образцы выделившихся газов, собранные на адсорбционных трубках, термически десорбированные в ГХ-МС
|
Профили газовых компонентов и кривые концентрации
|
Долгосрочные испытания материалов на выбросы, анализ следовых количеств
|
|
Испытание в камере (испытание в эмиссионной камере)
|
Образец помещают в герметичную камеру при фиксированной температуре для обнаружения выброса ЛОС
|
Уровни общего содержания летучих органических соединений (TVOC)
|
Оценка запаха для всего транспортного средства или его частей
|
|
Массив газовых датчиков (электронный нос)
|
Несколько датчиков имитируют обонятельные нервы человека для обнаружения и цифрового картирования запахов.
|
Цифровой профиль запаха, распознавание образов
|
Быстрый скрининг, автоматизированный контроль качества процесса запаха
|
|
Динамическая ольфактометрия
|
Образцы запахов разбавлялись и предоставлялись членам комиссии для определения порога обнаружения и статистики интенсивности.
|
Порог обнаружения запаха, индекс интенсивности
|
Борьба с городскими запахами, анализ источников промышленных запахов, выбор материалов
|
Как инженеры могут контролировать запах у источника?
Контроль запаха на этапе выбора материала — самая экономически эффективная и действенная стратегия.
Предлагаются следующие рекомендации:
|
Тип метода
|
Конкретная техника/метод
|
Принцип/Механизм
|
Применимые сценарии
|
|
Контроль источников материалов
|
Использовать высокочистое сырье и усовершенствовать процесс полимеризации |
Уменьшить количество остаточных мономеров, растворителей и примесей
|
Закупка сырья и разработка рецептур материалов на ранней стадии
|
|
Используйте добавки со слабым запахом (например, полимерные антиоксиданты)
|
Повышение устойчивости к миграции и окислительной деградации |
Инженерные пластики, детали автомобилей и бытовой техники
|
Закупка сырья и разработка рецептур материалов на ранней стадии
|
|
Оптимизация рецептуры
|
Добавить адсорбенты (например, активированный уголь, цеолит) |
Улавливать выделяющиеся газы
|
Системы смешивания пластиков и композитных материалов
|
|
Добавить дезодоранты (например, циклодекстрины)
|
Включать/комплексировать молекулы запаха для снижения летучести
|
Материалы для герметизации, упаковочные пленки, бытовые пластмассы и т. д.
|
Системы смешивания пластиков и композитных материалов
|
|
Оптимизация обработки
|
Применить вакуумную дегазацию, вторичную экструзию и сдвиговую вентиляцию
|
Снизить температуру/время обработки и улучшить высвобождение низкомолекулярных веществ
|
Производство экструзионных/литьевых профилей и инженерных пластиков
|
|
Очистка оборудования, предотвращение перекрестного заражения
|
Устранить остаточные «внешние источники запаха»
|
Сценарии смешанной обработки нескольких материалов |
Производство экструзионных/литьевых профилей и инженерных пластиков
|
|
Методы постлечебной обработки
|
Использовать термическую обработку (старение), фотоокисление и УФ-облучение
|
Способствовать раннему высвобождению или разложению остаточных малых молекул |
Детали салона автомобиля, композитные панели, изделия из кожи
|
|
Обработка поверхности (например, плазма, покрытие)
|
Изменить поведение поверхностной эмиссии и адсорбции
|
Детали с покрытием и текстурированные декоративные поверхности
|
Детали салона автомобиля, композитные панели, изделия из кожи
|
|
Структурное проектирование
|
Оптимизировать толщину материала и геометрическую структуру
|
Снизить уровень выбросов на единицу площади
|
Корпуса электронных приборов, панели управления автомобильных центров и другие области, требующие проверки на близком расстоянии.
|
Революция отбора от «обонятельного опыта» к «молекулярному дизайну»
Снижение уровня запаха в салоне автомобиля — это не просто вопрос сенсорной оптимизации, но и системный инженерный подход, охватывающий химию полимеров, кинетику массопереноса и аналитическую химию.
Для инженеров по выбору материалов важно установить корреляцию между «структурой – эксплуатационными характеристиками – запахом»:
Когда регулярность молекулярной цепи ПП увеличивается на
15%
, выделение альдегидов можно уменьшить путем
38%
;
Когда молекулярная масса пластификаторов ПВХ увеличивается с
300 Да до 500 Да
, скорость миграции уменьшается на
60%
.
Такая логика проектирования на молекулярном уровне является ключом к преодолению технологического «узкого места» в области производства материалов со слабым запахом.